intellectual property rights
The Article 13 Express: WHA 77.1 – WHO Pandemic Agreement
On Wednesday, 17 April 2024 at 15:25 CEST, the WHO secretariat circulated an advance, unedited English version of the proposed draft WHO Pandemic Agreement; the WHO secretariat also circulated the accompanying draft resolution, WHA77.1 WHO Pandemic Agreement. For KEI’s analysis of the 16 April 2024 Pandemic Agreement draft, please see: KEIcomment16April2024draftWHOpandemictreaty.
The eight page draft resolution presents a two-track approach for the path forward in the implementation of the proposed WHO Pandemic Agreement. Operative paragraph 13 of WHA77.1 states:
PART 7: Mandate to D-G/Secretariat
13. REQUESTS the Director-General, with regard to Article 6.5 (Preparedness, readiness and resilience), Article 7.3 (Health and care workforce), Article 10.6 (Sustainable and geographically diversified production, and technology transfer and know-how), Article 13.1 (Supply chain and logistics), Article 13.6 (vaccine and therapeutic related compensation and liability during pandemics), Article 14 (Regulatory strengthening), and Article 20.3 (Coordinating Financial Mechanism) of the Agreement, to implement with immediate effect the activities under those aforementioned Articles attributed to the WHO Secretariat, and report thereon to the Seventy-eight World Health Assembly, as well as, following the entry into force of the Agreement, all sessions of the Conference of the Parties and of Committee E of the World Health Assembly;
On 19 April 2024, the WHO secretariat convened an informal WHA77 pre-meeting for Member States, non-State actors in official relations, and the WHO Secretariat. At this informal, Knowledge Ecology International posed a question to the WHO Secretariat regarding the reference in operative paragraph 13 of WHA77.1 to Article 10.6 (Sustainable and geographically diversified production, and technology transfer and know-how.
![]()
In the 16 April 2024 draft of the WHO Pandemic Agreement, Article 10.6 does not exist.
![]()
The WHO secretariat clarified my question about the reference in operative paragraph 13 of WHA77.1 to Article 10.6 (Sustainable and geographically diversified production, and technology transfer and know-how; the Secretariat acknowledged the reference to Article 10.6 was a drafting error and would be fixed.
Operative paragraph 13 of WHA77.1 refers to the immediate effect articles: “to implement with immediate effect the activities under those aforementioned Articles attributed to the WHO Secretariat, and report thereon to the Seventy-eight World Health Assembly”. The “immediate effect” articles of the WHO Pandemic agreement (16 April 2024 version) include: Article 6.5 (Preparedness, readiness and resilience), Article 7.3 (Health and care workforce), Article 13.1 (Supply chain and logistics), Article 13.6 (vaccine and therapeutic related compensation and liability during pandemics), and Article 14 (Regulatory strengthening), and Article 20.3 (Coordinating Financial Mechanism) of the Agreement. Based on the secretariat’s clarification, it is likely that the corrected version of WHA77.1 will also list Articles 10. Sustainable and geographically diversified production, and 11. Transfer of technology and know-how as “immediate effect” articles.
While the provisions referred to in paragraph 13 of the Pandemic agreement resolution direct the WHO Director-General to implement them with immediate effect, the draft resolution envisions 3 intergovernmental working group (IGWG) negotiations for the following topics: 1) Conference of the Parties (COP IGWG), 2) WHO Pathogen Access and Benefit-Sharing System (PABS IGWG), and 3) One Health approach (OH IGWG) to be dealt with in a slow stream. For One Health and PABS, the resolution proposes that the PABS system and the One Health instrument/s be operational no later than 31 May 2026. With respect to the Conference of Parties, the resolution proposes that the COP IGWG “shall be held no later than 1 December 2025.” At the 19 April 2024 informal, WHO acknowledged that some Member States had indicated that they would prefer the establishment of just one IGWG process to handle the COP, One Health, and PABS workstreams.
The post The Article 13 Express: WHA 77.1 – WHO Pandemic Agreement appeared first on Knowledge Ecology International.
KEI comments on the 16 April 2024 draft of the WHO Pandemic agreement
On Friday, 19 April 2024, Knowledge Ecology International (KEI) published these comments on the 16 April 2024 draft of the WHO Pandemic agreement.
KEIcomment16April2024draftWHOpandemictreaty
The 16 April 2024 text (A/INB/9R/3, Proposal for the WHO Pandemic Agreement) can be found here:
DRAFT_WHO-Pandemic-Agreement_16-April-2024-1
The post KEI comments on the 16 April 2024 draft of the WHO Pandemic agreement appeared first on Knowledge Ecology International.
WIPO SCCR 45: Brazil’s opening statement
On Monday, 15 April 2024, Ambassador Guilherme de Aguiar Patriota (Permanent Representative of Brazil to the World Trade Organization and other economic organizations based in Geneva) delivered the following opening statement (in Brazil’s national capacity) at the 45th session of WIPO’s Standing Committee on Copyright and Related Rights (SCCR).
45 SCCR
Opening statement
Thank you, Madam Chair,
Brazil would like to congratulate you and the vice-presidents for your election.
I would also like to thank the Secretariat for the work in preparation of this session and for its openness to suggestions and comments presented by the Brazilian delegation during this process.
On the Broadcasting Organizations Treaty, Brazil can constructively engage in the negotiation of a signal-based treaty, in line with what was agreed during the 33rd WIPO General Assembly.
Nevertheless, the “slightly modified” third revised draft under consideration – document SCCR/45/3 – still requires considerable revision, notwithstanding more than 25 years of discussions. Some of its persistent shortcomings include, but are not limited to:
1) the definition of “broadcasting organizations” and of program-carrying signal, which do not contemplate Members, such as Brazil, which would be ready to update protection only with respect to the rights of traditional broadcasting organizations over their transmissions, to the extent that these rights are established under applicable legislation.
2) the fact that the draft-treaty’s own provision on “Limitations and Exceptions” prevents these from being fully enforceable.
3) the language on “Obligations Concerning Technological Measures” in relation to Limitations and Exceptions, which does not comply with the Marrakesh treaty, representing an unwarranted regressive trend.
4) The lack of clarity in respect of the object of protection, whether the program-carrying signals or the Broadcasting Organizations themselves, as wording changes depending on different provisions.
5) The extension of exclusive rights beyond the linear program transmissions of broadcasting organizations, encompassing pre-broadcast signals, post-fixation transmissions by any means, the transmission of stored programs by any means, retransmissions by any means.
6) The draft creates new layers of protection in favor of broadcasting organizations, on top of the existing copyrights of authors, performers, and producers of phonograms. It is not clear how this would benefit the latter rights holders, in spite of such claim being claim made in the fourth preambular paragraph.
We have spent too much time over the years trying to move forward a discussion and a
negotiating process that simply have not achieved meaningful convergence. Until they do, my delegation is of the opinion that we should not have this matter as a permanent item of the SCCR, taking up two full days of a five-day SCCR session, when the Committee has so many other fundamental issues to discuss, such as the digital agenda and AI.
For Brazil, the possibility of progressing on a draft Broadcasting Organizations Treaty requires focusing on a signal-based treaty only, not currently the case.
_____
On the agenda item dealing with Exceptions and Limitations, Brazil welcomes the results achieved so far, namely the Work Program proposed by the African Group and adopted at the 43rd session of the Committee. It is crucial to protect and promote the interests of the wider public to access science, technology, and culture. Brazil is ready to constructively engage to develop a strong Implementation Plan for the Work Program on L&E.
____
Furthermore, Brazil stresses the urgency for this Committee to properly address Copyright in the digital environment. A Draft Work Plan has been submitted by GRULAC. Previous documents presented by the regional group, as well as studies prepared by the Secretariat have underlined the difficulties facing rightsholders in enforcing their rights in the digital environment. This issue requires appropriate attention and time allocation within the SCCR, and justify holding two Committee Sessions per year, a tradition my delegation strongly supports.
GRULAC’s Work Plan provides a timeline for the elaboration of studies, the organization of regional and multilateral discussions, and the review of national, regional and international practices. These activities would support a debate on the need for an international legal framework around copyrights in the digital environment, without prejudging its nature. The topic is proposed as a permanent agenda item of the SCCR, reflecting the huge challenges digitalization poses to artists, authors, and related rights holders.
It is worth noting that AI would greatly increase the importance of the proposed digital debate and should be tackled in detail under the SCCR. We welcome the presence of Brazilian artists in the “Information Session on the Opportunities and Challenges Raised by Generative AI as it Relates to Copyright” organized as part of this session.
We look forward to discussions that address both opportunities and challenges arising from the growing use of AI in content creation. The Committee should debate recent disputes involving creators and AI companies, examine regulatory proposals about transparency and fair remuneration for creators in the AI context, as well as the protection of voice, image, and other personal attributes, as part of performing rights. This is a unique opportunity to reaffirm the strategic relevance of the SCCR within the global Copyright system, as AI and other contemporary trends of the digital age challenge the foundations of Bern Convention’s human-centric dimensions.
Thank you.
The post WIPO SCCR 45: Brazil’s opening statement appeared first on Knowledge Ecology International.
The WHO pandemic treaty: The Peace Clause and its discontents
On Tuesday, 2 April 2024, Politico published the onscreen text WHO pandemic treaty text of Wednesday, 27 March 2024; the time stamp of this 110 page text is 12:44 CET. Article 11 of the proposed agreement contains provisions on transfer of technology and know-how. Nestled within article 11 is paragraph 4bis, the peace clause. The 27 March 2024 pandemic treaty text can be found here: https://keionline.org/misc-docs/who/inb9.wed.27march.pdf

Article 11.4 states:
The Parties that are WTO Members recognize that they have the right to use to the full, the flexibilities inherent in the TRIPS Agreement as reiterated in the Doha Declaration on the TRIPS Agreement and Public Health of 2001, which provide flexibility to protect public health including in future pandemics, and shall fully respect the use thereof by others.
Article 11.4bis (the peace clause) states:
[4bis. The Parties shall not challenge, or otherwise exercise any direct or indirect pressure on the Parties that undermine the right of WTO Members to use TRIPS flexibilities at any multilateral, regional, bilateral, judicial or diplomatic forum. (BRA, COL, GTM, SLV, NIC,TUN, ARG, AG + EGY, BGD, FJI, PHL, PAK, IDN) (DEL EU, JPN, USA, UK, NZL, ROK,CAN, CHE)]
A pandemic accord armed with such a peace clause would set an important norm buttressing countries’ sovereign right to use TRIPS flexibilities “at any multilateral, regional, bilateral, judicial or diplomatic forum” without the specter of “direct or indirect pressure”. At the WHO pandemic treaty negotiations, the proponents of the peace clause include: Brazil, Colombia, Guatemala, El Salvador, Nicaragua, Tunisia, Argentina, the African Group + Egypt, Bangladesh, Fiji, Philippines, Pakistan, and Indonesia. The opponents of a peace clause in the WHO Pandemic Accord are: the European Union, Japan, the United States of America, the United Kingdom of Great Britain and Northern Ireland, Canada, and Switzerland.
On Tuesday, 2 April 2024, an informal was convened to resolve the profound differences remaining in Article 11; KEI was informed that Article 11.4bis remained a divisive issue.
Ellen ‘t Hoen, Director of Medicines Law & Policy, provided the following expert insight:
The proposed peace clause in the pandemic accord in fact echoes the basic principle of the WTO TRIPS Agreement. Article 1.1 of the TRIPS Agreement specifies that countries are not obliged to adopt TRIPS-plus measures and “shall be free to determine the appropriate method of implementing the provisions of this Agreement [TRIPS] within their own legal system and practice”. In other words, the proposed peace clause is a welcome reminder of this basic principle, certainly in the context of pandemics.
James Love of KEI made this observation:
In 2001 the WTO Adopted the Doha Declaration on TRIPS and Public Health, and paragraph 4 of that agreement stated that WTO members “should” implement the exceptions in intellectual property laws ” to promote access to medicines for all.” Various versions of this have been adopted in a variety of multilateral, plurilateral and bilateral agreements, but there has persisted enormous pressures on developing countries when they consider actually granting compulsory licenses or using other flexibilities. Here in an agreement dealing with pandemics and emergencies, an agreement motivated in no small part by the inequality of timely access that was a huge feature of the COVID 19 experience,you find several high income countries opposing an agreement to simply honor and give effect to the 2001 WTO agreement on TRIPS and Public Health, and this says volumes about the lack of solidarity and the extent of pharma industry control of the negotiations in those countries.
This is paragraph 4 from the 2001 WTO Doha Declaration on TRIPS and Public Health.
4. We agree that the TRIPS Agreement does not and should not prevent members from taking measures to protect public health. Accordingly, while reiterating our commitment to the TRIPS Agreement, we affirm that the Agreement can and should be interpreted and implemented in a manner supportive of WTO members’ right to protect public health and, in particular, to promote access to medicines for all.
In this connection, we reaffirm the right of WTO members to use, to the full, the provisions in the TRIPS Agreement, which provide flexibility for this purpose.
Luis Villarroel, Director and founder of Innovarte stated:
The “rightly called peace clause” is a proposed solution to the problem of pressures from other countries against developing countries attempting to implement TRIPS flexibilities within their national laws. Such pressures, which also originate from dominant pharmaceutical companies, hinder their capability to adopt flexibilities that are used by developed countries, like the USA. Those are certainly needed for protecting public health in case of a pandemic. The peace clause is a reaffirmation of the principle of sovereignty. It is very discouraging that the European Union, Japan, the United States of America, the United Kingdom of Great Britain and Northern Ireland, Canada, and Switzerland have opposed to such a clause.
KM Gopakumar, Legal Advisor of Third World Network (TWN) observed:
There is well-documented evidence that direct and indirect pressure is used by developed countries and big pharmaceutical corporations to prevent countries from using TRIPS flexibilities, especially government use/compulsory licenses. This proposal makes such coercive activities clearly illegal and allows countries to make use of the TRIPS flexibilities to fulfil. their obligations on the right to health. This is important to facilitate equity.
Brook Baker, Senior Policy Analyst, Health GAP, and Professor Northeastern U. School of Law provided the following comment:
Not only is the idea of a “peace clause” against foreign pressure to restrict adoption, use, and protection of TRIPS flexibilities enshrined in Art. 1.1 of the TRIPS Agreement and in the Doha Declaration, it is absolutely an essential element of a meaningful Pandemic Treaty. The proposed “peace clause” would free countries from the fear of retaliation if and when they take appropriate measures to ensure timely, adequate, and affordable suppliers of pandemic-related health technologies, including from local producers. It is deeply hypocritical for the U.S to oppose a “peace clause” when it issued dozens of contractual, government-use licenses to its suppliers of COVID-19-related health products. It is equally hypocritical of the E.U. to oppose such a guarantee at the same time it is legislating a regional-wide compulsory license system for future pandemic-related health technologies. Likewise, Japan, the U.K., Switzerland, Canada, Australia, and New Zealand also all have compulsory license legislation and would cry bloody murder if any other country challenged their sovereign decision to issue a compulsory license if they were undersupplied in a future pandemic. Why is it too much to ask that there are codified assurances that rich countries will respect a trade-threat ceasefire during future pandemics when countries use lawful TRIPS flexibilities?
Peter Mayburduk, Director of Public Citizen’s Access to Medicines Group observed:
We are disappointed in the Biden administration for failing to support developing country partners in their pursuit of access to medicines for all and the use of health rights consistent with WTO rules. This is an opportunity to show overdue respect and indicate that Washington is open to modest course adjustments in support of a meaningful negotiation. To conclude a meaningful pandemic accord, every country must be prepared to revisit some of its outdated prejudices.
Hu Yuanqiong,PhD, Senior Legal and Policy Advisor for Médecins Sans Frontières (MSF) Access Campaign, provided the following response:
Governments of developing countries often face unilateral political pressure when utilising IP flexibilities to address national health needs. For example, the United States Trade Representative’s Special 301 Report consistently criticises the implementation of IP rules and practices promoting access to medical products in various countries. In recent years, in a departure from convention, the report has recognised countries’ right to use compulsory licenses as a public health safeguard to overcome IP barriers on medical products. However, the report continues its public criticism of countries’ use of other TRIPS flexibilities, including stricter patentability criteria to check evergreening, stringent patent examination guidelines and restrictions on data and market exclusivities. Back in 2016, the UN Secretary General’s High Level Panel on Access to Medicines recommended in its report that governments must refrain from explicit or implicit threads, tactics and strategies that undermine the use of TRIPS flexibilities. In light of protecting the right to health as its ultimate objective, the ongoing INB negotiation provides an opportunity to address this long-term issue by explicitly requesting governments to refrain from putting pressures that could undermine the use of TRIPS flexibilities.
Viviana Munoz Tellez, PhD. Coordinator of the Health, Intellectual Property and Biodiversity Programme, South Centre, stressed:
The Peace Clause is necessary. Developed countries and their pharma industry apply undue pressure and threat of litigation to keep developing countries from adopting pro-public health measures that are compatible with the WTO TRIPS agreement (see SC Research paper 132 by Carlos Correa https://www.southcentre.int/research-paper-132-june-2021/).
Dr. Mohga Kamal-Yanni MPhil. MBE, Senior policy advisor to The People Vaccine Alliance, provided the following insights:
How many times did we hear Northern politicians talk about “Equity and solidarity” during the COVID crisis? Yet they acted for inequity and disunity. They protected only their citizens and the profiteering of pharmaceutical companies, at the expense of the rest of the world. The South was forced into a COVID vaccine apartheid.
The Pandemic Accord offers an opportunity to protect every human being, irrespective of who they are and where they are. To do so, it must include solid commitments for technology transfer and removing intellectual property barriers. Unsurprisingly however, rich Northern countries are continuing to refuse to act and are instead committed to protecting pharmaceutical companies’ monopoly power to decide on production, allocation, and price of key lifesaving medical technologies for potential pandemics.
Northern refusal to include a clause that reaffirms what the South already has as a right under the TRIPS agreement, is another glaring example of Northern disregard of people in the South. If they do not agree, then Southern countries must disregard northern pressure and move together to use the TRIPS flexibilities without waiting for approval from pharmaceutical companies or Northern countries. It is time for the South to recognise its power and for the North to realise that medical technology is a global public good that should benefit all that need it.
I hope that the North stops talking about equity because as the Egyptian saying goes: “I hear what you say, I believe you, but I see what you do and I am puzzled”.
Patrick Durisch, health policy expert at Public Eye, commented:
It is appalling that Switzerland opposes a clause already enshrined in a 2001 WTO Agreement on TRIPS and Public Health, even more so in a negotiation over a pandemic accord. For Switzerland and its pharma industry, dealing with pandemics and emergencies is like “business as usual”. The Swiss government already exercised undue political pressure in the past during episodes of compulsory licenses involving Swiss pharma companies in Colombia and Thailand, and it is constantly trying to undermine TRIPS flexibilities through bilateral free trade agreements such as in the recent EFTA-India trade deal. Switzerland should stop overprotecting its pharma industry and instead focus on equity and protect the right to health.
Jaume Vidal, Senior Policy Advisor at Health Action International (HAI) provided the following response:
The so-called peace clause is not only symbolic; it goes to the very root of an effective and equitable response to pandemics beyond legal and regulatory constraints. The opposition by the EU and others to explicitly forbid reprisals against governments because of health-related measures is ominous and concerning; it should be redirected in order to uphold European values of equity, solidarity, as well to respect and promote fundamental human rights to life, health and human dignity.
Piotr Kolczyński, EU Health Policy & Advocacy Advisor for Oxfam commented:
While TRIPS flexibilities may look equal on paper for all WTO members, the imbalance of power in global trade and IP rights’ ownership makes their practical application by higher- and lower-income countries incomparable. Decades of experience show that lower-income countries using flexibilities, such as compulsory licenses, face huge commercial and political pressure or even threats of trade sanctions not to do so.
The WHO Parties’ commitment in the Pandemic Accord not to in any way obstruct or seek to dissuade others from making full use of existing flexibilities could significantly improve the practical ability to exercise these rights in the global South.
Ella Weggen, Senior Global Health Advocate, Wemos provided these insights:
The Peace Clause is nothing new, because it recognises the right to use existing TRIPS flexibilities. But it is very necessary to prevent countries from blocking the use. It underlines the legal possibilities that countries have, to use TRIPS flexibilities. It is disappointing that Northern countries oppose this, especially the EU. During the Covid-19 pandemic, several (EU) countries underlined the importance of global access to Covid-19 innovations. The EU opposed the so-called TRIPS waiver, but instead several countries, like the Netherlands, indicated the importance of the already existing TRIPS flexibilities and the need for facilitating their use. The Peace Clause that is being negotiated as part of the Pandemic Accord would underline this. It is appalling that the EU is opposing it, which shows how powerful the lobby of pharmaceutical companies is.
During the Covid-19 pandemic, Wemos advocated strongly for the sharing of intellectual property, knowledge and data, but this did not happen voluntarily on a large scale. Governments should have the ability to impose measures when their public health is threatened, and the use of TRIPS flexibilities makes that possible. The peace clause therefore seems like a no-brainer. So why are so many rich countries reluctant? Protection of profits over public health? History will repeat itself again and again, looking at the inequality of access in the time of Covid, if we do not change the status quo and facilitate the use of TRIPS flexibilities.
Vanessa López, Executive Director of Salud por Derecho, offered these comments:
Agreed in 2001 and part of the TRIPS agreement, the peace clause is out of the question and its presence in the Pandemic Accord a guarantee of equity and solidarity. Hence, the position of northern countries against the use of flexibilities shows once more the little learned during the COVID crisis. It is very disappointing to see the EU opposing these basic instruments which could contribute to better access to health technologies to low- and middle-income countries, while the region is reviewing its current legislation and mechanisms to reload for future health public crises. The vision of business as usual has no place in the Pandemic Accord since we have seen the consequences. This must be the cornerstone of this accord.
Christopher Baguma, Director of Programs at Ahaki, expressed these observations:
Amidst the chaos of global health crises, the peace clause in the WHO Pandemic Treaty negotiations stands as a beacon of stability, ensuring equitable access to essential medicines and healthcare resources for all nations, regardless of their economic status or geopolitical power.
The Peace Clause is an opportunity to further clarify the use of intellectual property flexibilities, especially during pandemics. By ratifying the use of TRIPS flexibilities during public health emergencies while recognizing the import of opposition, the challenges that have for long been faced by developing countries in utilizing the flexibilities would be minimized. Moreover, ratification of such a clause is fully aligned with public health considerations, as prioritized by the WTO TRIPS Agreement and the Doha Declaration on the TRIPS Agreement and public health.
The post The WHO pandemic treaty: The Peace Clause and its discontents appeared first on Knowledge Ecology International.
INB9: KEI written statement
INB 9
Written statement by Knowledge Ecology International
28 March 2024
Transparency
The negotiations on a WHO pandemic agreement should be more transparent. The negotiating texts with attributed country positions should be public and the meetings should be webcast. These are reasonable expectations. The World Intellectual Property Organization (WIPO) operates in this way; it even allows stakeholders to listen to informal negotiations on texts and has demonstrated an ability to adopt treaties on topics of considerable controversy and with commercial consequences.
There is considerable paranoia and misinformation about the WHO in social media, and the unnecessary secrecy of this negotiation is not helpful. While the texts are often leaked, both industry and several well-informed civil society groups have access, and some can afford to attend the negotiations, the general public is locked out. The lack of transparency erodes confidence in the WHO in general and the pandemic agreement in particular.
In the negotiating text, KEI had hoped there would be an article or chapter on transparency, and a commitment to implement WHA72.8 on transparency, and we urge delegates to protect and enhance the several areas where transparency is currently mentioned.
Sanctions
A number of countries currently face economic and technology sanctions. It is generally recognized that such sanctions should not extend to the supply of medical products, or the financing, transport, or other services required to provide those products. We are disappointed that some WHO members in this negotiation have opposed language proposed as Article 13.1bis that would ensure that the humanitarian exceptions to sanctions are effectively implemented.
Technology transfer
There is clearly an interest among negotiators to facilitate the scale-up and decentralization of the manufacturing of pandemic-related countermeasures in an emergency, but also sharp divisions among the parties about how to address this need.
Going forward, consideration should be given to an additional type of pooling approach, one that more closely resembles some industry pools, or some pooled procurement mechanisms, and which can provide stronger incentives to share technology by limiting the benefits of the pool to parties that opt-in and meet certain obligations for membership.
One such pooling agreement could involve rights in government-funded R&D, particularly the rights to use inventions, regulatory data, know-how, and access to cell lines and other biologic resources.
In addition to the pooling of rights from public sector-funded R&D, there could be technology buyout pools, with the benefits of the buy-outs being limited to the parties paying for the buyouts.
The requirements to join any of these pools would be scaled according to the incomes and stage of development of the members, and while the benefits of the pools would be limited to the members, every state would have an opportunity to contribute and join the pool.
The proposal should be seen as a complement to other measures on technology transfer and not a substitute.
The post INB9: KEI written statement appeared first on Knowledge Ecology International.
Brackets on March 23, 2024 INB text on Chapter 11
To get an idea of the challenges of getting agreement on the WHO pandemic accord text, consider the state of just one article on Saturday, March 23, 2024. Article 11 has 6 paragraphs on technology transfer. According to an analysis by Arianna Schouten, by the end of the day on Saturday, 50 countries or groups have at least one bracket in the Article. The European Union has 49, the United States has 43. There are a total of 579 country positions recorded. Arianna looked at the brackets and judged 112 to be obviously pro-pharma and 183 to be obviously pro-access.
The text of Chapter 11, published today by Politico (not the WHO), is here:
POLITICO-23march2024-inb9-chapter11
Arianna’s counts of the brackets is here.
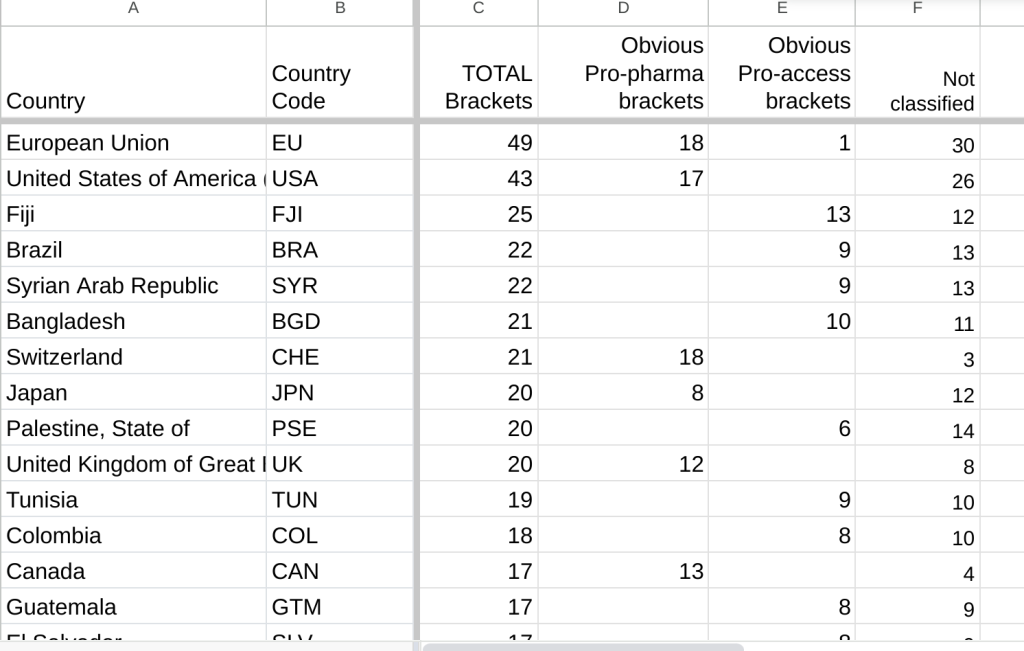
The post Brackets on March 23, 2024 INB text on Chapter 11 appeared first on Knowledge Ecology International.
INB 9 – Oral statement by Knowledge Ecology International
On Monday, 18 March 2024, Knowledge Ecology International (KEI) delivered the following oral statement (in person) at the 9th meeting of the World Health Organization’s (WHO) Intergovernmental Negotiating Body (INB).
With respect to Article 11.2, the current references to “to all developing countries” are not optimal if you want to encourage more sharing of technology.
While soft obligations to share technology with developing countries are helpful and appropriate, creating mechanisms where countries have a self-interest to cooperate should also be included.
One approach that could have a higher ceiling on what technology is shared is an opt-in pool, where the benefits of the pool are only available to countries that opt-in.
To opt-in, parties would agree to some combination of terms and conditions on government funded R&D, levels of relevant R&D funding, and conditions on procurement.
The post INB 9 – Oral statement by Knowledge Ecology International appeared first on Knowledge Ecology International.
WGIHR 7 (8 March 2024): KEI oral intervention
On Friday, 8 March 2024, Knowledge Ecology International (KEI) delivered the following intervention at the resumed session of the seventh meeting of the Working Group on Amendments to the International Health Regulations (2005).
8 March 2024
The two negotiations are efforts to formalize a collaboration among governments to deal with pandemics and health emergencies. There are some measures that will be required to be undertaken by all parties, and other measures where a universal mandate will not be possible.
An important challenge is to find ways to share access to the inventions, know-how and other inputs to drugs, vaccines and other countermeasures.
One approach is to create incentives for parties to share some rights acquired from publicly funded R&D or procurements, in a reciprocal manner, with parties that also share. Another approach would be to provide money or other incentives to acquire rights to patented inventions, know-how and other inputs, from private rights holders.
This does conflict in some ways with the notion that the benefits would be universal. It can be implemented so that the collaboration is open to any party.
The reason for sharing rights only among parties that opt-in, is to create an incentive to share, and to address the sharing of the costs as well as the benefits of product development. In such a collaboration, the obligations on parties, involving both terms and conditions in funding and procurement agreements and funding of R&D or product acquisition should scale with incomes and stages of development.
Any reciprocal sharing of rights should be without prejudice to other efforts to obtain more universal sharing of rights.
The post WGIHR 7 (8 March 2024): KEI oral intervention appeared first on Knowledge Ecology International.
Request for Amendment to add Risdiplam for Spinal Muscular Atrophy (SMA) to Schedule 1 of the Canadian Patent Act
On March 1st, Knowledge Ecology International sent a letter to The Hon. Mark Holland, Ministry of Health, and The Hon. François-Philippe Champagne, Minister of Innovation, Science, and Industry, urging the prompt consideration of adding risdiplam, a drug to treat spinal muscular atrophy (SMA), to Schedule 1 of the Canadian Patent Act.
The three approved treatment for spinal muscular atrophy, Zolgensma, Spinraza and Evrysdi, are all marked by excessive pricing. This has led to disparate access globally, with even high-income countries unable to provide access.
In the letter, KEI asks for the timely response from the Ministers to the following actions:
-
- A meeting to discuss the issue and plan next steps; and
- A prompt recommendation from the Ministers of Health and Innovation for the addition of risdiplam to Schedule 1; and
- An order from the Governor in Council to amend Schedule 1 within the next 60 days.
The letter can be accessed here: Request for Amendment to Schedule 1 of the Patent Act
The post Request for Amendment to add Risdiplam for Spinal Muscular Atrophy (SMA) to Schedule 1 of the Canadian Patent Act appeared first on Knowledge Ecology International.
Save the Date – 21 February 2024– EU and US state practice in the Covid-19 response: National law and policy improvements and their relevance to WHO Pandemic Treaty negotiations
Knowledge Ecology International (KEI) invites delegates attending the 8th meeting of the WHO Intergovernmental Negotiating Body (INB) to a side event on Wednesday, 21 February 2024. This event is hybrid. For registration, please contact thiru [at] keionline.org.
Title: EU and US state practice in the Covid-19 response: National law and policy improvements and their relevance to WHO Pandemic Treaty negotiations
Date: Wednesday, 21 February 2024
Time: 18:00 CET to 20:00 CET
Speakers include:
Dr Ellen ‘t Hoen – Director, Medicines Law & Policy
James Love, Director, Knowledge Ecology International (KEI)
As the WHO pandemic treaty negotiations unfold, key policy tensions remain on provisions involving the transfer of technology and know-how, access and benefit sharing, financing, and attaching conditionalities to publicly funded research and development including pandemic countermeasures.
Meanwhile, several countries and regions continue to improve national and regional laws and policies to ensure the safeguarding of public health, including ongoing efforts to streamline compulsory licensing in the EU, the intensive application of government use during COVID by the US government, and the recent development of clarifying the use of march-in rights.
These developments in the EU and the US provide significant reference points for the WHO INB negotiations. The side event aims to introduce the recent major developments and practices in the US and the EU concerning access to medicines, R&D conditions and intellectual property, and to discuss the relevance of these developments to the ongoing INB negotiations in the WHO.
A recording of the event can be found here.
Ellen ‘t Hoen’s presentation can be found here: https://medicineslawandpolicy.org/wp-content/uploads/2024/02/Ellen-t-Hoen-intervention-21-Feb-INB8.pdf
James Love’s presentation can be found here:
USA, non-voluntary-use-patents-INB, 2024

The post Save the Date – 21 February 2024– EU and US state practice in the Covid-19 response: National law and policy improvements and their relevance to WHO Pandemic Treaty negotiations appeared first on Knowledge Ecology International.